CredibleMeds® Process for Assigning Risk
ADVERSE DRUG EVENT CAUSALITY ANALYSIS (ADECATM)
CredibleMeds.org was created in 2000 under a federal grant from the Agency for Healthcare Research and Quality and since 2012 is maintained by AZCERT. AZCERT clinical scientists designed ADECATM, a risk stratification process which they use to assess whether drugs can cause QT prolongation and/or torsades de pointes (TdP).
The ADECA process is described in detail in the journal Drug Safety Vol. 40(6): 465-474. 2017 and can be accessed at:
http://link.springer.com/article/10.1007/s40264-017-0519-0
The process includes application of the Bradford Hill criteria to the available laboratory and clinical evidence for drug safety.1-4 The process is shown graphically in Figure 1.
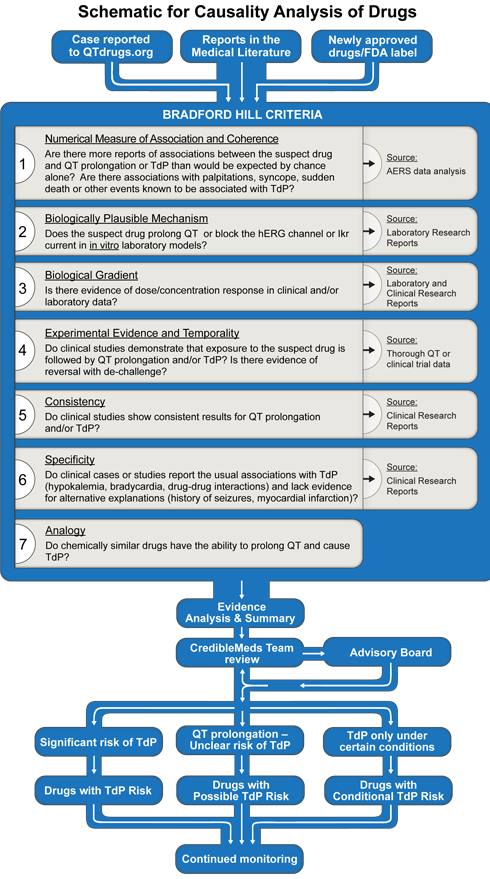
Specific drugs come to CredibleMed's attention by three general pathways:
- a question or case is received at www.QTdrugs.org or www.CredibleMeds.org,5
- evidence of potential risk is found in a monthly literature search for publications reporting blockade of hERG or IKr potassium channels, QT prolongation or TdP or
- a drug's FDA-approved label includes mention of "QT prolongation" or "TdP".
The Bradford Hill criteria,1 listed in figure 1, are applied to the basic science and clinical evidence that are obtained from the medical literature (PubMed search), the FDA label, the FDA's summary basis of approval (if posted on the FDA's website), an analysis of the adverse events reported to the FDA's Adverse Event Reporting System (AERS) and reports or inquiries to CredibleMeds. In some cases, laboratory or clinical experiments are performed by AZCERT investigators to fill gaps that augment the analysis.6-14
Analysis Tools: The AERS database
AERS is a computerized database of adverse events (AE) reported for drugs and biologics that is hosted by FDA as a post-marketing safety surveillance tool known as MedWatch. The FDA's AERS data are made publically available quarterly and are interrogated by members of the CredibleMeds team using the Empirica SignalTM software (Oracle Health Sciences), a data mining tool initially developed for use by FDA scientists.15
While AERS data are extremely useful and have been the FDA's basis for detecting many serious ADRs, it has limitations that have been well described.16 For example, because AERS does not contain measures of overall exposure to each drug, counts of drug-AE combination frequencies must be viewed as numerator-only data, without denominators that would provide risk ratios.For CredibleMeds analysis purposes, AE counts are compared to expected counts, computed under a model where exposures and AEs are either independent, or conditionally independent considering other covariates. This approach, termed a disproportionality analysis, generates the ratios of observed-to-expected counts for drug-AE combinations and yields relative reporting ratios.17
Statistical Analysis of AERS Data for Potential Adverse Event (AE) Signals
The CredibleMeds team uses two approaches to mine the AERS database for potential signals associated with specific drugs or drug combinations. The Multi-Item Gamma Poisson Shrinker (MGPS) approach is a way of finding unexpectedly frequent associations between AE and a drug) in the AERS database. It uses the Empirical Bayesian Geometric Mean (EBGM) to estimate the relative reporting ratio17 with a 90% credible interval (CrI). The lower and upper bounds of the Crl are designated as EB05 and EB95 respectively. In this approach, a signal is considered relevant if the EBGM and the entire 90% CrI are greater than 2.00 (i.e. EB05>2.00).
Secondly, a logistic regression (LR) approach is used to model how the probability of an event depends on multiple predictors. Such predictors can be the presence or absence of each of several specified concomitant drugs in reports of TdP, or a mixture of interacting drugs (concomitant QT-prolonging drugs or a drug plus a metabolic inhibitor), and other covariates such as patient gender, age, reporting year, etc. In this way, the disproportionality estimates can adjust for these or other covariates as well as concomitant drugs.
One of the advantages of logistic regression is that the logistic odds ratios (LOR) for each factor are represented by the exponentials of the regression coefficients. This is advantageous for interpretation purposes, since the odds ratio is a commonly used measure of association in the medical literature. For the purpose of these analyses, an arbitrary (but more conservative) threshold of 2.0 is used for the entire 90% confidence Interval (CoI) of the LOR estimated by LR.
Figure 2 demonstrates the type of data display generated by the Empirica analysis for drugs. The drug in this case is methadone, a drug whose risk of TdP was identified because of cases reported to the CredibleMeds team10 and is now included in the list of drugs "with known risk" of TdP.
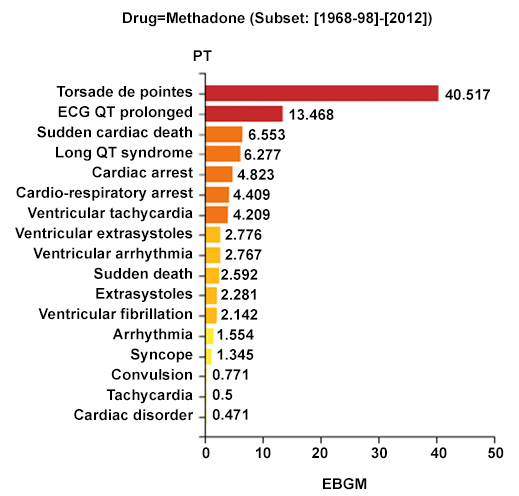
Figure 2. Comparative signals for seven adverse events of interest associated with methadone. EBGM: Empirical Bayesian Geometric Mean; EB05-EB95: 90% Credible Interval (CrI) for the EBGM; bar lengths represent observed EBGM magnitudes; error bars represent 90% CrI; color shades represent magnitude-categories for EB05.
Risk Stratification: The CredibleMeds QTDrugs List (previously known as QTdrugs.org Drug Lists)
To evaluate a drug's risk, the following elements (underlined) in the Bradford Hill causality analysis are assessed by the CredibleMeds team: The Numerical association of a drug with reports of QT prolongation or TdP is estimated by signal strength in the AERS database by computing the EBGM, with its 90%CI (i.e. EB05-EB95), as explained above using the Empirica SignalTM (Oracle Health Sciences) software. Coherence refers to similar signal strength with reports in AERS or other sources of related events (QT prolongation, cardiac arrest, ventricular tachycardia, syncope etc.).18Plausibility is influenced by whether a drug has a relevant pharmacologic action that is known to cause TdP (e.g. hERG or IKr blockade).18Biological gradient means the dose or exposure-response relationship. Experimental evidence consists of in vitro data, clinical trials, and challenge/re-challenge. Temporality considers the interval between drug administration and subsequent development of TdP. Consistency comes from consistent trends in AERS and across reports in the scientific literature. Specificity relates to identifiable factors known to predispose to TdP, e.g. hypokalemia, extreme bradycardia or genetics.19Analogy includes assessing similar examples with other drugs that have structural similarities.
The ADECA process has rigorous requirements for the type of evidence that must be positive in order for a drug to be placed in one of the three risk categories shown in Figure 3 below. The specific requirements are indicated by the checked boxes and described in greater detail below.
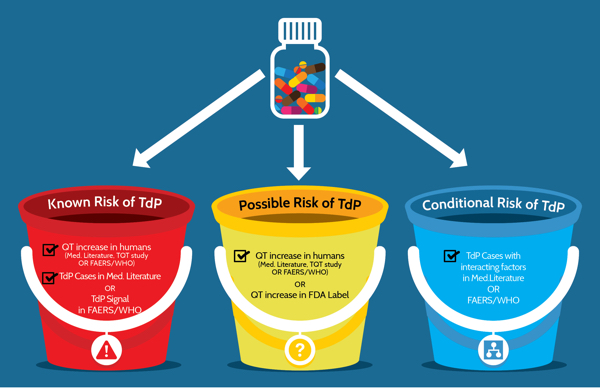
Figure 3: Three categories of risk and the type of evidence that is required for drugs that prolong the QT interval and/or cause TdP. FAERS/WHO: FDA Adverse Event Reporting System and World Health Organization's Vigibase of adverse event reports for drugs.
Evidence Requirements for Risk Categories
Figure 4 (below) lists the 16 types of evidence used by AZCERT and how, if positive, the evidence can fulfill requirements or can support the placement of a drug into one of the three primary risk categories.
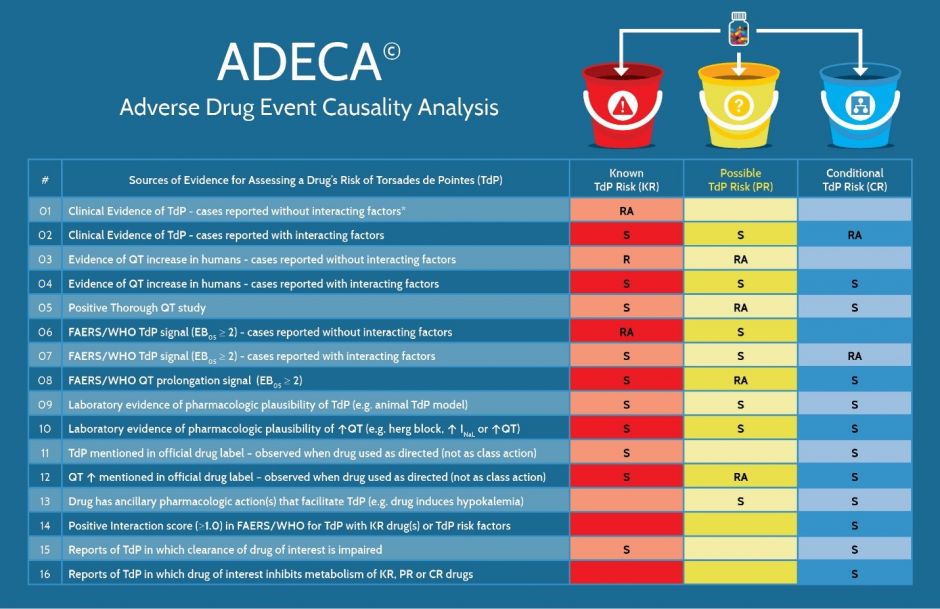
Figure 4. The types of evidence that are required or used to support placement of a drug into one of the risk categories for QT prolongation and/or TdP. R = Required, RA = Required, with Alternative and S = Supportive. * Interacting factors include concomitant use with KR, PR or CR drugs, metabolic inhibitors, hypokalemia, hypomagnesemia, bradycardia, excessive dose or congenital Long QT syndrome
As shown in Figure 5, each risk category has certain types of evidence that are required (R) to be positive whereas certain other types of positive evidence are considered to be only supportive (S). Also, some types that are labeled RA are considered to be equivalent and any one of these can be accepted as a required alternative (RA). For example, in order for a drug to be placed in the Known Risk category there must be consistently positive evidence of TdP (with the caveat that the drug was used as directed in labeling) and that the evidence must be either 1) cases reported in the published medical literature or 2) a positive signal for TdP in FAERS or Vigibase. Also, drugs in the Known Risk category and those placed in the Possible TdP Risk category require consistently positive evidence of QT prolongation from either 1) the published medical literature, 2) a thorough QT study, 3) the drug label or 4) a positive signal for QT prolongation in FAERS or Vigibase. For the Conditional TdP Risk category, there are also alternatives for the types of TdP evidence but the preponderance of case reports or the evidence must include the presence of confounding or interacting factors such as concomitant use with drugs that are metabolic inhibitors of QT prolonging drugs, overdose, hypokalemia, hypomagnesemia, bradycardia or a diagnosis of congenital Long QT Syndrome.
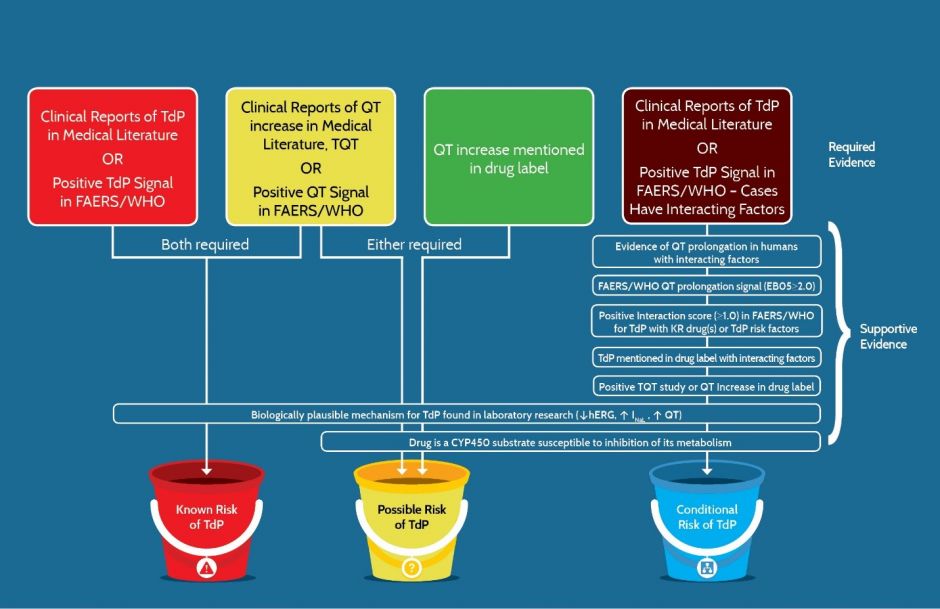
Figure 5. The types of evidence that are either “Required” or “Supportive” for a drug to be placed in one of the three risk categories for QT prolongation and/or TdP. FAERS/WHO: FDA Adverse Event Data System or World Health Organization’s Vigibase of adverse event reports.
Using these criteria, a systematic assessment of the available data supporting potential changes to the lists is conducted and a formal evidence analysis is generated. The analysis is reviewed at a regular weekly meeting of the AZCERT Team and if a recommendation to change one of the lists is unanimous, the change is made and the Advisory Board is notified. If any member of the 34 member Advisory Board raises a question, the decision is reevaluated and may be presented to the entire Board for discussion and reconsideration. If the initial recommendation for change is not unanimous by the AZCERT Team, the decision is referred to the Advisory Board along with the evidence analysis. If the recommended change is approved by the Advisory Board, the website is updated and changes made to one of the following lists within 24 hours:
Risk of TdP: Substantial evidence supports the conclusion that these drugs prolong QT intervals and have a risk of TdP when used as directed in labeling.
Possible risk of TdP: Substantial evidence supports the conclusion that these drugs can cause QT prolongation but there is insufficient evidence that the drugs, when used as directed in labeling, have a risk of causing TdP.
Conditional risk of TdP: Substantial evidence supports the conclusion that these drugs prolong QT and have a risk of developing TdP but only under certain known conditions (e.g. excessive dose or overdose, or being the index or interacting agent in a drug-drug interaction).
Drugs that should be avoided, if at all possible, by patients with congenital Long QT Syndrome (CLQTS):
- To be maximally safe, this list includes all drugs that are in any of the three lists above.
- Drugs are also included on this list if they pose a unique risk to this patient population with congenital long QT, e.g. central nervous system (CNS) and adrenergic stimulants, even though they may not prolong the QT interval, per se.
Medications on all lists are continuously monitored for emerging relevant evidence. In recent years, the lists have been revised approximately once every 30-45 days as shown in figure 6.
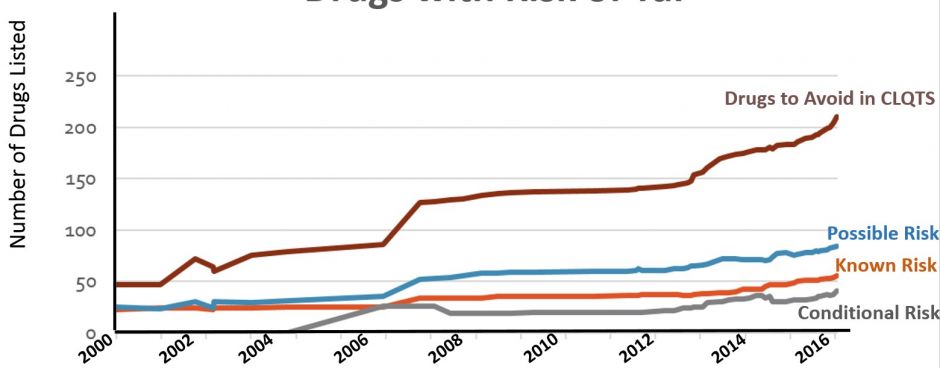
Figure 6. Number of drugs on the QTdrugs List from 2000-2016 in each of the four risk categories
The list can be searched by brand or generic name, sorted according to drug class, therapeutic use, etc and downloaded and printed.

- HILL AB. The environment and Disease: Association or causation? Proc R Soc Med 1965;58:295-300.
- Shakir SAW, Layton D. Causal association in pharmacovigilance and pharmacoepidemiology: thoughts on the application of the Austin Bradford-Hill criteria. Drug Safety: An International Journal of Medical Toxicology and Drug Experience 2002;25(6):467-471.
- Perrio M, Voss S, Shakir SAW. Application of the bradford hill criteria to assess the causality of cisapride-induced arrhythmia: a model for assessing causal association in pharmacovigilance. Drug Safety: An International Journal of Medical Toxicology and Drug Experience 2007;30(4):333-346.
- Woosley RL. Discovering adverse reactions: why does it take so long? Clin Pharmacol Ther 2004;76(4):287-289.
- Remich SA, Kilborn MJ, Woosley RL. The role of internet-based registries in tandem with genetic screening for the study of drug-induced arrhythmias. Current Therapeutic Research 2002;62(11):787-795.
- Shin JG, Kang WK, Shon JH et al. Possible interethnic differences in quinidine-induced QT prolongation between healthy Caucasian and Korean subjects. Br J Clin Pharmacol 2007;63(2):206-215.
- Katchman AN, Koerner J, Tosaka T, Woosley RL, Ebert SN. Comparative evaluation of HERG currents and QT intervals following challenge with suspected torsadogenic and nontorsadogenic drugs. J Pharmacol Exp Ther 2006;316(3):1098-1106.
- Pearson EC, Woosley RL. QT prolongation and torsades de pointes among methadone users: reports to the FDA spontaneous reporting system. Pharmacoepidemiol Drug Saf 2005;14(11):747-753.
- Curtis LH, Ostbye T, Sendersky V et al. Prescription of QT-prolonging drugs in a cohort of about 5 million outpatients. Am J Med 2003;114(2):135-141.
- Kornick CA, Kilborn MJ, Santiago-Palma J et al. QTc interval prolongation associated with intravenous methadone. Pain 2003;105(3):499-506.
- Katchman AN, McGroary KA, Kilborn MJ et al. Influence of opioid agonists on cardiac human ether-a-go-go-related gene K(+) currents. J Pharmacol Exp Ther 2002;303(2):688-694.
- Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA 2001;285(10):1322-1326.
- Shuba YM, Degtiar VE, Osipenko VN, Naidenov VG, Woosley RL. Testosterone-mediated modulation of HERG blockade by proarrhythmic agents(1). Biochem Pharmacol 2001;62(1):41-49.
- Benton RE, Sale M, Flockhart DA, Woosley RL. Greater quinidine-induced QTc interval prolongation in women. Clin Pharmacol Ther 2000;67(4):413-418.
- Szarfman A, Machado SG, O'Neill RT. Use of screening algorithms and computer systems to efficiently signal higher-than-expected combinations of drugs and events in the US FDA's spontaneous reports database. Drug Saf 2002;25(6):381-392.
- Szarfman A, Tonning JM, Doraiswamy PM. Pharmacovigilance in the 21st century: new systematic tools for an old problem. Pharmacotherapy 2004;24(9):1099-1104.
- Almenoff JS, DuMouchel W, Kindman LA, Yang X, Fram D. Disproportionality analysis using empirical Bayes data mining: a tool for the evaluation of drug interactions in the post-marketing setting. Pharmacoepidemiol Drug Saf 2003;12(6):517-521.
- Drew BJ, Ackerman MJ, Funk M et al. Prevention of torsade de pointes in hospital settings: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. Circulation 2010;121(8):1047-1060.
- US Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationfor
PatientsandProviders/ucm111350.htm , 2011.








